Professor Tien-Lu Cheng, Director of Research and Development at Kaohsiung Medical University (KMU), has led his team to develop the world’s first Universal Antibody Lock, a groundbreaking technology designed to reduce the side effects associated with antibody drugs. The innovation allows an antibody to remain “locked” in healthy tissues and only become “unlocked” and activated in disease-specific regions. This selective activation mechanism not only significantly lowers adverse effects but also greatly improves patients’ quality of life.
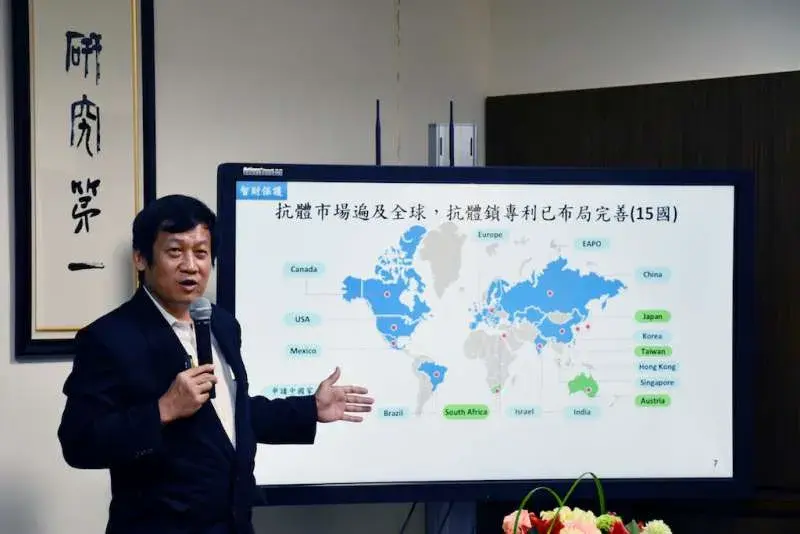
KMU President Yu-Chih Chung emphasized that this globally unique invention by Professor Cheng and his research team has already earned the 10th National Innovation Award. The team has also filed patents in 15 countries and secured approvals in Taiwan, South Africa, Australia, Japan, and more. The research was additionally published in the renowned international journal PLOS Biology in mid-June. This breakthrough stands to benefit patients requiring long-term antibody treatments by alleviating drug-related discomfort and toxicities.
Professor Cheng noted that while antibody drugs are among the most widely used and successful modern therapeutics—with an annual global market exceeding USD 100 billion—about 24% of antibody candidates fail clinical trials due to severe side effects. The Antibody Lock developed by the KMU team has the potential to “revive” these abandoned drugs, turning them into safer next-generation therapeutics. For example, patients with rheumatoid arthritis often require lifelong medication and face risks such as severe infections (e.g., tuberculosis, bacterial or fungal diseases), hepatitis reactivation, and increased cancer incidence. Incorporating the Antibody Lock into such treatments could substantially reduce these adverse effects.
Seven Years of Cross-Disciplinary and International Collaboration
The team further demonstrated the ability to customize the Antibody Lock for different antibody drugs, making it applicable across the global antibody therapeutics market. This versatility has already attracted strong interest not only from Taiwanese pharmaceutical companies but also from industry partners in the United States and Japan, with commercial potential estimated to exceed USD 10 billion.
The KMU research team comprises 30 members spanning biomedical sciences, biotechnology, genetic engineering, and clinical medicine. Over the course of seven years, they invested heavily in the research—spending hundreds of thousands of NT dollars on laboratory mice and more than NT$2 million to ship antibodies overseas for efficacy and safety testing at an animal research center in Greece. Through extensive interdisciplinary and international collaboration, the team successfully completed the development of the world’s first Universal Antibody Lock, marking a major scientific milestone for both KMU and Taiwan.